Exploring the Therapeutic Potential of Ibogaine for Traumatic Brain Injury (TBI) in Special Operations Forces Veterans: A Groundbreaking Research Study
Magnesium-Ibogaine: The Stanford Traumatic Injury to the CNS (MISTIC) Protocol - A Pilot Study in Special Operations Veterans with Traumatic Brain Injuries (TBI)
Dr. Williams and researchers in his lab completed a pioneering research study of the use of ibogaine to treat post-traumatic stress disorder (PTSD) caused by traumatic brain injuries in U.S. Special Operations Forces Veterans. Published in Nature Medicine, researchers found that ibogaine, a plant-based psychoactive compound, when combined with a cardioprotective agent, safely led to improvements in depression, anxiety and functioning among veterans with traumatic brain injuries.
TBI is the “signature injury” of the Iraq and Afghanistan conflicts, with nearly half of veterans (46%) screening positive for TBI. Conventional treatment may not always offer the desired relief to these individuals.
Ibogaine is a naturally occurring psychoactive substance. On average, treatment with ibogaine led to significant improvements in functioning, PTSD, depression and anxiety. The effects persisted until at least one month after treatment, the endpoint of the study. Our study is in its early stages; however, initial results from our observational study lead us to believe it holds the potential to offer new treatment options for those whose lives are profoundly affected by TBI and its debilitating consequential effects.
Antidepressant Effects of Ketamine Linked to the Opioid Receptors in the Brain
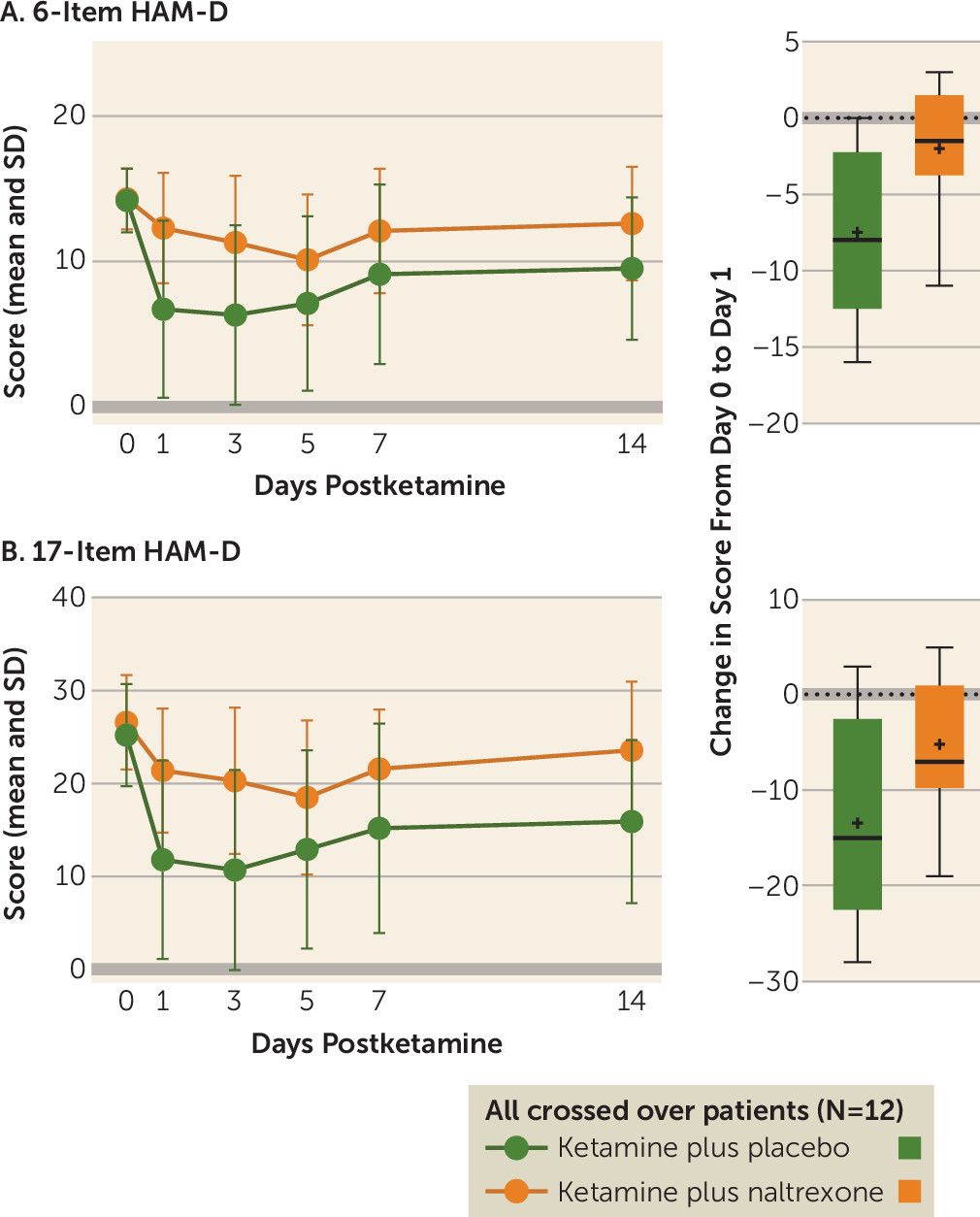
According to a study published in the American Journal of Psychiatry on August 29, 2018, researchers at Stanford uncovered that ketamine functions as an antidepressant by activating the brain’s opioid system. This discovery challenges prior assumptions that the antidepressant properties of ketamine were solely attributed to its influence on the glutamate system. The conventional understanding of ketamine’s antidepressant effects had led to its widespread use for depression treatment and the development of glutamate-blocking drugs as antidepressants.
Furthermore, this new revelation sheds light on the intricate interplay between depression, pain, and opioid addiction. It presents an opportunity for healthcare professionals to reconsider their approaches to treating these three interconnected conditions.
In a secondary analysis of the clinical trial published in Molecular Psychiatry, the team tested whether naltrexone lessens the anti-suicidality effects of ketamine. The participants were pre-treated with naltrexone or a placebo prior to intravenous ketamine in a double-blinded crossover design. Results indicate that opioid receptor activation plays a significant role in the anti-suicidality effects of ketamine.